Brown Invents
The pursuit of more stable and powerful lithium-ion batteries hinges in part on the development of improved electrolytes. Current lithium-ion batteries contain electrolytes made from lithium salt dissolved in a liquid organic solvent. Liquid electrolytes can short circuit and are made with chemicals that are toxic and flammable. Solid electrolytes are made of ceramic, and while excellent at conducting ions, they are thick, rigid, and brittle.
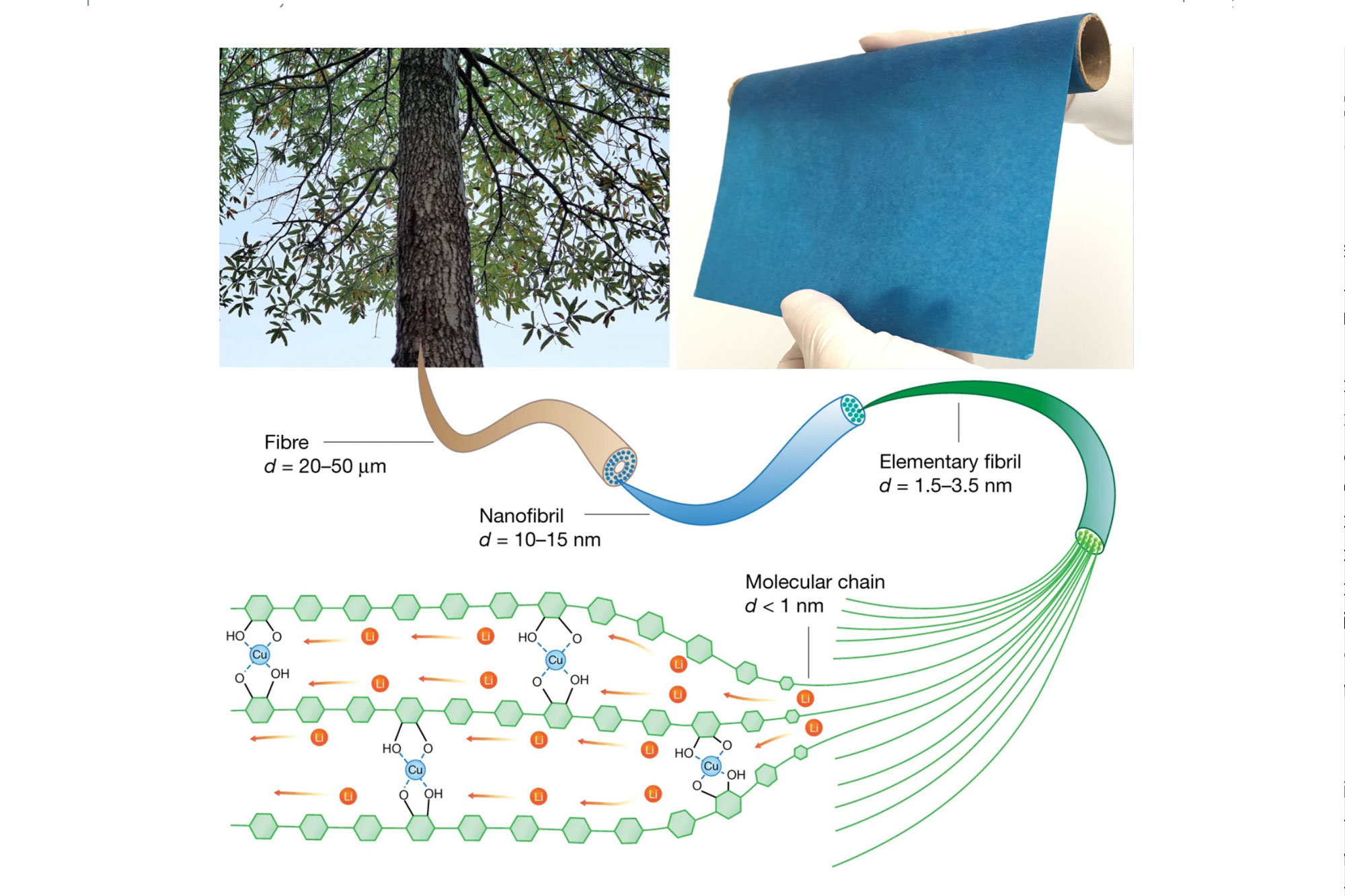
Now there’s a better option—a thin and flexible material derived from trees for use in solid-state batteries. The new material was developed by a team of researchers co-led by the laboratory of Yue Qi, a professor in Brown’s School of Engineering, and a materials science laboratory at the University of Maryland. In a paper published in Nature in October 2021, the team describes a solid ion conductor that combines copper with cellulose nanofibrils— polymer tubes derived from wood. The paper-thin material, which has an ion conductivity of 10 to 100 times that of other polymer ion conductors, could be used as either a solid battery electrolyte or as an ion-conducting binder for the cathode of a solid-state battery. Eventually, the new material could be a step toward bringing solid-state battery technology to mass production.
