A More Equitable Way to Analyze DNA
New methods will improve understanding of how genetic conditions affect different populations.
New methods will improve understanding of how genetic conditions affect different populations.
By using new methods for analyzing DNA data and medical records, researchers from Brown University are helping improve the understand- ing of complex traits that will make more discoveries relevant to nonwhite, non-European ancestry groups.
Genome-wide association (GWA) datasets, which are commonly used by geneticists, are based on the assump- tion that individual genetic mutations underwrite the genetic basis of traits, explained Sohini Ramachandran, a professor of biology and computer science who directs both the Center for Computational Molecular Biology and the Data Science Initiative at Brown. The idea is that a discovery about those mutations will be relevant to all people across a range of diverse ancestry groups, so if the finding is used to develop treatments for genetic conditions, it will be applicable for all people with that condition.
However, recent studies have shown that GWA results estimated from self-identified European individuals are not transferable to non-European individuals. Consequently, the insights from GWA datasets are largely biased toward individuals with European ancestry.
“ It’s really important to us that we understand trait architecture better so that we can make steps towards provid- ing effective therapies for everyone, from every ancestry group ”
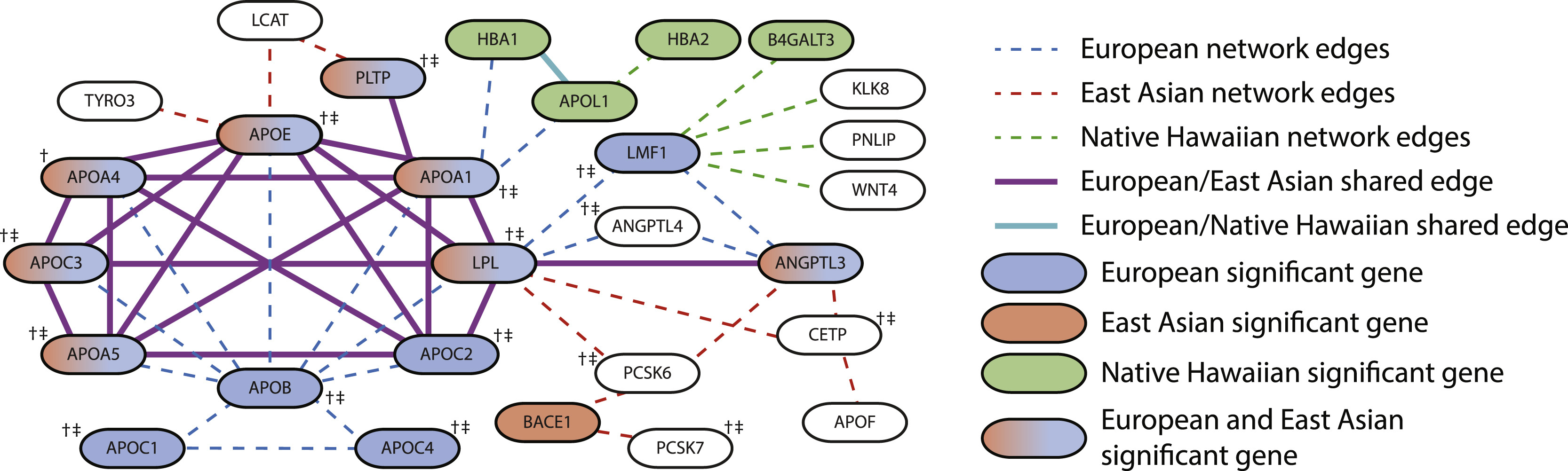
Ramachandran teamed up with Brown assistant professor of biostatistics Lorin Crawford and former PhD student Samuel Pattillo Smith to analyze a ream of data across multiple biobanks using a new enrichment analysis. This more expansive methodology, which was previously developed in a collaboration between Ramachandran and Crawford to address bias and underrepresentation, moves beyond individual mutations to include genes and pathways.
In a study published in the American Journal of Human Genetics, the researchers illustrated examples of the robust associations of trait determinants, or patterns of similarity, while studying 25 traits in over 600,000 individuals from seven diverse human ancestries. A majority of these would not have been identified using GWA alone, the researchers said.
With these similarities, discoveries about the nature of diseases or illnesses and their responses to potential treatments have become more relevant to larger groups of people, including populations that have previously been ignored or understudied.
In a field like genomics, the stakes are high, Ramachandran said.
“It’s really important to us that we understand trait architecture better so that we can make steps towards provid- ing effective therapies for everyone, from every ancestry group,” she said.