Discoveries in Aging Research
From cell studies to therapeutics to elder care, Brown researchers tackle the aging process.
From cell studies to therapeutics to elder care, Brown researchers tackle the aging process.
The indignities of aging creep in slowly. A bit of wrinkled skin, a nagging pain in your joints, a blurry tinge to formerly perfect vision. Age-related quirks like these are a normal part of living—but, strangely, they don’t affect us all the same way. For some, ailments may be just an annoyance; for others, they’re significant enough to require full-time care.
Why is this? If all factors are equal, how can a 70-year-old man be bedridden while the 95-year-old woman next door is still alert and active? A small army of research- ers at Brown is working on answers. On lab benches, in clinical trials, and in programs aimed directly at older adults, these scientists reveal how our bodies age and what we can do to prevent the worst effects of that aging process. With each new finding, they make painstaking, incremental progress, laying the foundation for our old age to become healthier and happier.
Basic Science
The Aging Cell
The work many Brown scientists are doing on aging falls squarely into the category of basic science, research that may not have an immediate application but that reveals the fundamental principles and phenomena that make our cells tick. It lays the groundwork for applied science by providing knowledge other researchers can build upon down the road. Bit by bit, these scientists are discovering how aging occurs on a cellular and molecular level and are creating the foundation for new drugs and other treatments in the future.
Part of the Package
Erica Larschan, associate professor of molecular biology, cell biology, and biochemistry, studies chromatin, the molecular “packaging” that holds our DNA. Under normal circumstances, she said, chromatin molecules wind specific stretches of DNA tightly around themselves, which prevents certain genes from being read. In doing so, chromatin is a critical part of regulating gene expression in a cell—but when it falters, the whole finely-tuned system can come crashing down.
“The packaging of the genome just starts to fall apart with age,” Larschan said. “That could be one of the first triggers or signals that drive the aging process, because once you dysregulate gene expression, it affects lots of other things in the body.”
Exactly why that happens is a central question of her research. Larschan wants to understand how chromatin changes in later life and how that change differs by sex. This trait could explain why women have higher rates of diseases like Alzheimer’s while men have higher rates of other diseases like amyotrophic lateral sclerosis (ALS).
She is just starting to investigate the root cause of these differences, but thanks to a new $12.5 million grant from the National Science Foundation to a project on which she is a co-principal investigator, she hopes to make significant progress in the next five years.
Cells in Stasis
Qian Chen, professor of orthopedic research and medical science, is also trying to figure out how aging affects individual cells. He focuses on osteoarthritis, a common ailment associated with aging and injury. Like Larschan, he thinks a breakdown in gene expression within cartilage cells—which may also be caused by damaged chromatin—could be a root cause of osteoarthritis.

Normally, Chen said, cartilage cells can divide a finite number of times. If joint tissue is damaged by injury or worn out by age, some cells hit their division limit before the wound is fully healed. Instead of just dying off like they usually would during development, a few of those cells settle into senescence, a lingering state of malaise where they can no longer divide yet refuse to go gently into that good night. These zombified cells hang out in joint tissue for years, causing long-term inflammation that could trigger arthritis.
“Once a few cells go senescent, you basically have a time bomb in your body. Even if you’re able to restore perfect mechanical function to an injured joint through surgery, those cells that are buried deep in cartilage will lead to osteoarthritis down the road,” said Chen.
To solve this problem, he is developing a delivery system for RNA therapies that could travel through cartilage, a notoriously dense and impenetrable substance. By creating a nanoparticle small enough to squeeze through its thicket of fibers, Chen’s work could lead to methods that treat osteoarthritis at the source and prevent inflammation from occurring.
Mixed Signals
Outside factors, like hormones and signaling molecules, also contribute to cellular aging. Professor of Biology Marc Tatar thinks that one of the body’s most ubiquitous hormones, insulin (which generally helps regulate blood sugars) could play a significant role.
For the past 20 years, Tatar has been studying how changes in insulin levels are linked to aging. Back in 2001, he found some tantalizing clues: by limiting insulin in fruit flies, he could prolong their lives by 30 to 40 percent. The longevity came at a cost, however—while the flies survived longer than their peers, they also had diabetes, stunted growth, and poor reproduction.
“ It’s hard to see the translation from fruit fly to human, but when you get down to the level of the cell, we’re all the same. ”
In 2022, Tatar and his team at Brown made a new and equally exciting discovery: a mutation in a tiny and understudied part of fruit flies’ insulin receptors that seemed to give them unusually long lives. Their lifespans stretched phenomenally, yet they could still grow, thrive, and reproduce like flies half their age with no adverse side effects.
The cause, Tatar believes, is the unique placement of the mutation. The slight tweak it created in the flies’ genetic code altered the structure of their insulin receptors in tiny ways that led to massive results. Some forms of insulin and its close cousins, insulin-like growth factors, could still bind normally to these mutated insulin receptors, preventing harmful side effects like diabetes, while other forms of the molecules were left out in the cold. Tatar suspects their absence is somehow responsible for the insects’ long lives.
Humans and fruit flies share many of the same genes that control insulin processes, so what’s good for these insects might also be good for people. If Tatar can show similar results in mammals, it could lead to a drug that combats the effects of old age.
“It’s hard to see the translation from fruit fly to human, but when you get down to the level of the cell, we’re all the same,” he said.
Without the incremental progress these scientists and their peers bring to the field, there would be no new medical insights, no drugs to combat diseases of aging, and no forward momentum in the field. Through their work with cells, genes, and molecules, these researchers are paving the way for the treatments of the future.
Yu-Wen Alvin Huang
Huang, assistant professor of molecular biology, cell biology, and biochemistry, studies the molecular basis of Alzheimer’s disease and examines how it differs from normal aging. He is currently focused on a protein called CHI3L1, which causes inflammation, stops immune cells in the brain from functioning normally, and prevents neuronal stem cells from producing more neurons. The protein may help trigger diseases like Alzheimer’s and other forms of dementia.
Nicola Neretti
Neretti, associate professor of molecular biology, cell biology, and biochemistry, is working to understand cellular senescence—a semidormant state that triggers widespread inflammation in the surrounding tissue—and is exploring its role in aging. His latest work examines how senescent cells accumulate in the body and contribute to age-related conditions such as cancer, heart disease, and Alzheimer’s.
Clinical and Applied Research
New Treatments and Therapies

Many researchers at Brown are already building on the work of their peers and are actively creating treatments for diseases of aging. These scientists are partnering with older patients
to conduct observational studies or are entering clinical trials for new drugs. Their innovative work could lead directly to therapies and diagnostic tools that will help us thrive well into our sunset years.
Early Action
William Heindel, professor of cognitive, linguistic, and psychological sciences, is one of those researchers. With his colleague, Senior Lecturer Elena Festa, he’s working on ways to spot neurological diseases like Alzheimer’s and other forms of dementia early in their course before patients show any symptoms. To do so, he’s using a series of deceptively simple computerized tests that can reveal if cognitive damage is happening in the brain even a decade or two before symptoms appear.
The tests challenge two components of our visual system that process what we see through two distinct neural pathways or “streams.” The first, called the dorsal stream, processes motion, luminance, contrast, and black-and-white elements of our vision; the second, called the ventral stream, handles object recognition and processes color. Both streams must work together seamlessly to coherently assess what we see.
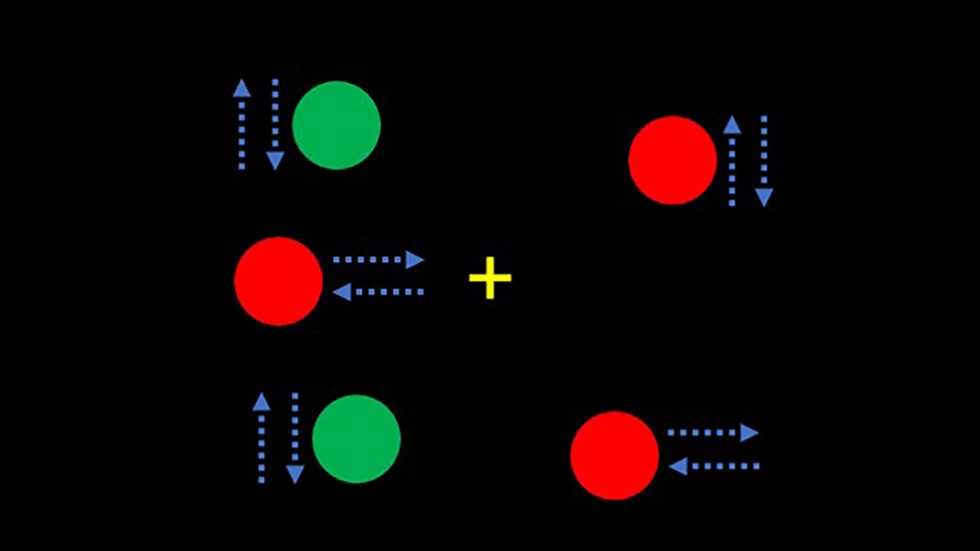
Heindel’s tests probe this connection. In the lab, a video screen shows many green dots moving left to right and red dots moving up and down, while a single green dot moves only up and down. Participants are asked to track this green dot with their eyes, and since doing so requires processing both motion (the dorsal stream) and color (the ventral stream), slight delays in their reaction, even just a by few milliseconds, can show that the link between both streams is breaking down.
“That’s the beauty of certain cognitive tests like this. You don’t necessarily need medical imaging to see what’s going on in the brain,” said Heindel.
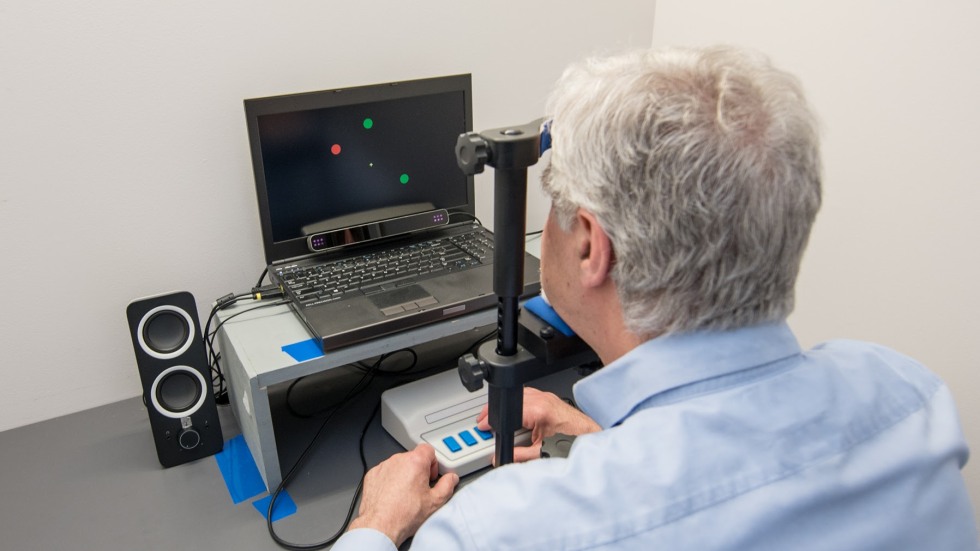
William Heindel demonstrates a cognitive test he developed with colleague Elena Festa, senior lecturer in cognitive, linguistic, and psychological studies, that can reveal whether cognitive damage is happening in the brain long before symptoms of neurological disease appear.
With their new imaging technique, the Lee lab generated an image of a brain microvasculature network in which different levels of betweenness, a key network property, were represented by a range of colors.
Replacing Neurons
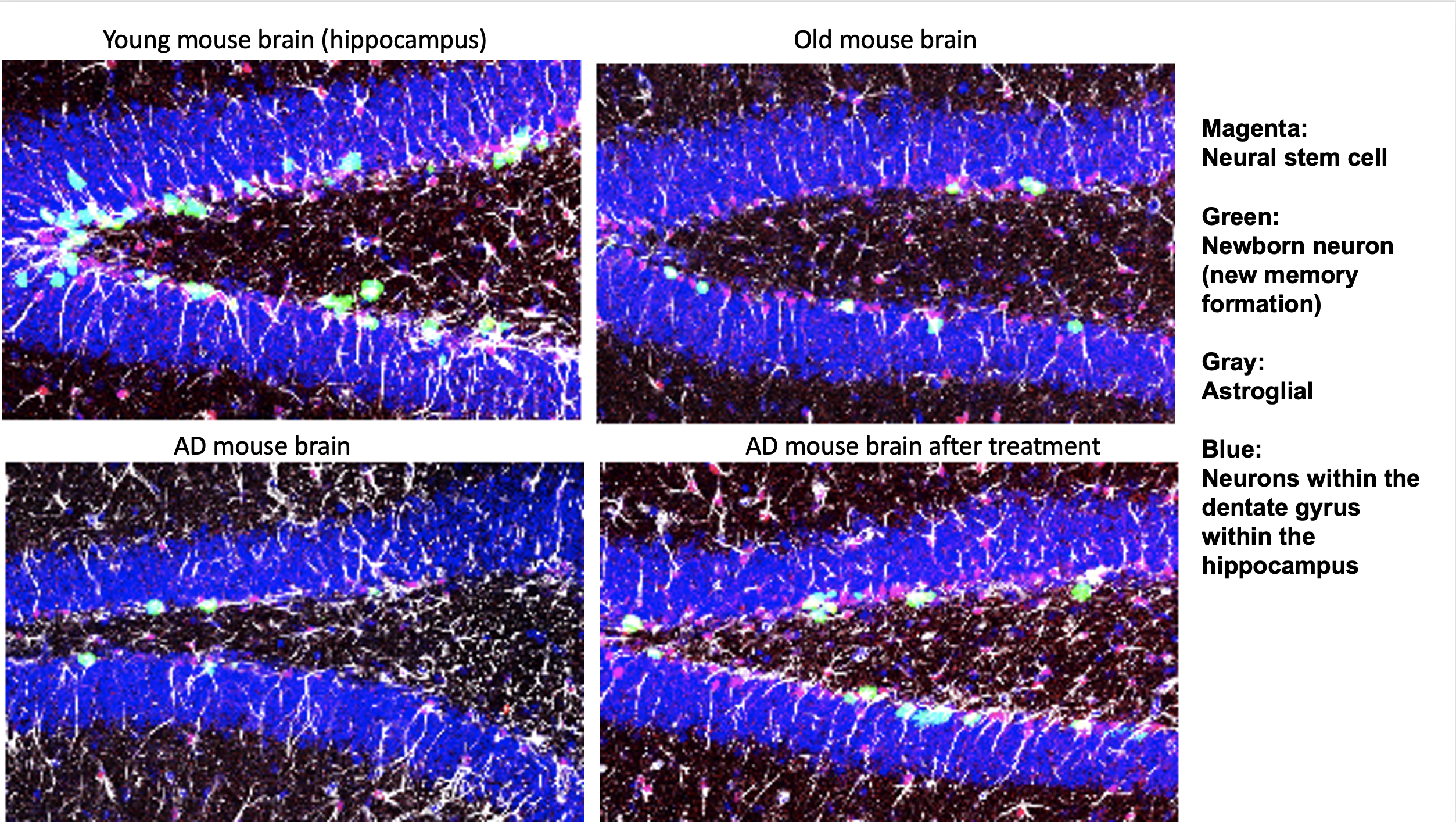
Spotting age-related disease is only half the battle; treating it is just as important. Justin Fallon, professor of neuroscience, and Ashley Webb, assistant professor of molecular biology, cell biology, and biochemistry, are working towards that goal along with Johnny Page, a former student of Fallon’s. Together they’ve created Bolden Therapeutics, a new biotech company based on their collaborative research.

Bolden aims to find treatments for various neurodegenerative diseases, from Alzheimer’s to ALS. These diseases kill off neurons in the brain through multiple means, leaving gaps in its complex circuitry. The team wants to replace missing neurons entirely to fix those broken connections, which could restore patients’ original brain function.
This may be possible by targeting stem cells in the hippocampus, a region of the brain that plays a central role in learning and memory, Webb said. Early in life, those cells crank out neurons regularly, but the older we get, the fewer they produce. A new therapy they are developing may offer a way to boost the activity of those cells on demand, creating new neurons whenever needed.
“That might be useful for normal cognitive decline as well as in situations like Alzheimer’s disease, which destroys cells in the hippocampus early on,” Webb said. “If we can figure out ways to replace the neurons that are lost in Alzheimer’s, that would be a game changer.”
Currently the researchers are testing their RNA therapy in mice and on human cells in the lab. If successful, the drug will move into clinical trials in the next few years, bringing the world closer to a treatment for millions of patients worldwide.
JonghwanLee.png)
Jonghwan Lee
Lee, assistant professor of engineering and brain science, is developing new methods for diagnosing Alzheimer's disease using advanced vascular imaging techniques. He is currently exploring how the degeneration of small blood vessels in the brain may contribute to cognitive decline and dementia. By better understanding this process, his work may lead to new strategies for preventing the disease, such as regular checkups for early detection through simple eye scans.
An Ounce of Prevention
Professor of Biology John Sedivy is also working on ways to treat age-related diseases in the brain. Instead of rebuilding damaged neurons, he’s focusing on a method to stop or slow that damage.
Sedivy, director of Brown’s Center on the Biology of Aging, studies cells that have entered senescence, a semidormant state that causes inflammation in surrounding tissue. If too many cells in the brain become senescent, he said, the resulting inflammation can damage or destroy neurons, causing diseases like ALS and dementia.

One cause of cell senescence in the brain may be retrotransposons, virus-like stretches of genetic code in our DNA. If activated, these bits of code can replicate themselves and damage cells. Sedivy’s lab discovered that cells also mistake retrotransposons for real viruses and mount antiviral defenses that fuel chronic inflammation. Through his biotech startup, Transposon Therapeutics, Sedivy is working towards a treatment that can stop that scenario from unfolding. He’s currently in early clinical trials for a new drug that can block retrotransposons in human brain cells and ultimately may prevent neurons from dying off in patients with ALS or Alzheimer’s.
“We’re discovering new things all the time,” he said. “Retrotransposons have been known for decades, but they haven’t attracted a lot of attention in the medical world. We’re really firmly connecting them with disease.”
Each of these researchers has a tangible sense that the process of aging is, essentially, the process of living. It’s an inextricable part of who we are; from the day we’re born, it’s a steady presence wherever we go. Just because our bodies are perpetually aging doesn’t mean we’re destined to end our days in illness, though: thanks to the clinical research Brown scientists are doing today, we may be able to stay healthy and mentally sharp late in life.

Hwamee Oh
Oh, associate professor of psychiatry and human behavior, is studying the early effects of Alzheimer’s disease pathologies, such as beta-amyloid plaques and tau-protein neurofibrillary tangles, on cognition and brain structure and function using advanced brain imaging techniques like MRI and PET. She is developing new cognitive tasks and methods for analyzing these scans to detect early cognitive and brain imaging signs of the disease and track its progression—work that could lead to earlier diagnosis and more effective treatments.
Gerontology and Elder Care
Improved Quality of Life
Most of us hope to live long lives, preferably in the comfort of our own homes. However, what that takes in practice isn’t so obvious, since assisted living facilities and nursing homes are full of older adults who require full-time professional care to meet their basic needs. A growing number of researchers at Brown are studying these care settings and finding that the key to healthy aging isn’t just medical intervention; it’s also the social and emotional framework
surrounding those patients. Issues like isolation, limited transportation, and poor access to nutritious food, they say, can be just as much of a problem as a chronic disease. So how can we know what kinds of care will give people the best possible outcomes?

Meals on Wheels recipient Lena Belton, a former army base supervisor and Sunday school teacher, says her favorite part of the meal delivery program is the volunteers who stop by to check on her.

Kali Thomas specializes in improving care for older adults needing long-term services and supports, such as Meals on Wheels.

Rosa Baier tests music and other nondrug health interventions to protect the autonomy of dementia patients

Erick Loucks researches the benefits of mindfulness practice on healthy aging, which include better cardiovascular health and cognition in older adults.
Sizing Up Care
These kinds of questions drive the work of Vincent Mor and Kali Thomas, professor and associate professor of health services, policy, and practice. Both are researchers at Brown’s Below: Meals on Wheels recipient Lena Belton, a former army base supervisor and Sunday school teacher, says her favorite part of the meal delivery program is the volunteers who stop by to check on her. Center of Gerontology and Healthcare Research (which Mor directed for 10 years), specializing in improving care for older adults.
“Nonmedical services, like meal prep, shopping, groceries, and laundry, are social determinants of healthy aging,” said Mor. “They are not historically the realm of medicine or healthcare. Yet they’re a critical part of making care more holistic so we can stop just treating one disease at a time.”

Mor and Thomas are combing through massive nationwide datasets, including Medicare electronic health records, to identify the best policies and practices for older adults. They’re searching for patterns that show which techniques and programs have genuinely effective results, and by quantifying those outcomes, they’re creating scientific evidence that could lead to more standardized and equitable care.
Thomas, for instance, is currently working with the nonprofit Meals on Wheels America to study how in-person meal deliveries impact recipients. She found that the meals themselves are only part of the benefit: social interaction with the person delivering them is also incredibly valuable for health outcomes. Thomas is testing that idea through a “pragmatic clinical trial”—one that takes place in the real world rather than in a clinic—and her results may help expand Medicare and private insurance coverage to include these services.
Pragmatic clinical trials are at the core of a nationwide effort to transform dementia care, led by Mor and Susan Mitchell, MD, professor of medicine at Harvard Medical School. Mor and Mitchell co-lead the NIA IMPACT Collaboratory, a research incubator evaluating nondrug, care-based interventions for dementia patients and their caregivers. The incubator is funded by a five-year $53.4 million grant from the National Institute on Aging awarded to Brown and Boston-based Hebrew SeniorLife in 2019, the largest federal award in Brown University history.
Finding What Works
Rosa Baier, professor of the practice of health services, policy, and practice, is also working to improve health interventions for older adults. Baier directs the Center for Long-Term Care Quality and Innovation at Brown. She partners with vast networks of caregivers representing more than 12,000 assisted living and long-term care facilities nationwide.
Through these partnerships, she and her colleagues have identified significant problems these facilities struggle with daily. Quality care for dementia patients, for instance, is an ongoing issue—in many cases, facilities manage those patients’ confusion and erratic behavior with unnecessary antipsychotic drugs.
Baier and her colleagues at the center are testing nondrug interventions that can both protect patients’ autonomy and aid in softening their outbursts. For example, one of the group’s current studies involves musical intervention, which involves working with caregivers to make personalized playlists that might help calm agitated dementia patients. The principal researcher on that work, Ellen McCreedy of Brown’s School of Public Health, has already helped more than 50 nursing homes develop a music-based regimen.
“These are exactly the kinds of studies where collaboration with the provider community is valuable,” Baier said. “There’s a synergy between the things that researchers are interested in and the things that address healthcare providers’ needs.”

Mind Over Body
Other innovations in elder care, like mindfulness practice, can also benefit healthy aging. Eric Loucks and Elena Salmoirago- Blotcher, MD, researchers at Brown’s Mindfulness Center and the Lifespan Cardiovascular Institute respectively, find that extended practice of those techniques can benefit cardiovascular health and improve cognition in older adults.
In a 2020 study, Loucks, associate professor of behavioral and social sciences, gathered a cohort of more than 200 older adults with elevated blood pressure and conducted an eight- week intensive mindfulness training program with them all. Under his guidance, the cohort learned to focus on how they felt when eating certain foods, exercising, taking medications, or consuming alcohol.
“ For patients with heart failure, this self-awareness is really important. Being able to notice if your legs are getting swollen, or if you are feeling more tired than usual, or if you are suddenly short of breath when you climb the stairs could help people seek medical attention and receive adequate treatment sooner rather than later. ”
After six months, he saw a marked improvement in the group. Participants were more likely to eat heart-healthy foods, reported lower levels of stress overall, and, most importantly, had a notable five-millimeter drop in average blood pressure.
Salmoirago-Blotcher, associate professor of psychiatry and human behavior, is studying other ways mindfulness practices may improve health and well-being in aging adults. She’s found that it can improve cognitive function in older people with heart failure or at risk for dementia. She is now conducting a more extensive study to see how mindfulness training can affect cognition. The study will also examine its effects on interoception, or awareness of what’s happening inside one’s body.
“For patients with heart failure, this self-awareness is really important. Being able to notice if your legs are getting swollen, or if you are feeling more tired than usual, or if you are suddenly short of breath when you climb the stairs could help people seek medical attention and receive adequate treatment sooner rather than later,” she said. No matter where we’re living in our
No matter where we’re living in our old age, the work of these scientists is ensuring that we'll be better cared for, more comfortable, and more aware of our health—all elements that will vastly improve our quality of life.
Rich Jones
Jones, professor of neurology, studies “cognitive reserve” in older adults, or how well their brains compensate for declining cognitive function in Alzheimer’s disease, other forms of dementia, and clinical aging. He is also exploring potential interventions for cognitive loss, such as exercise and mental training, that may help slow down or prevent these age-related changes.
Stephen Salloway, MD
Salloway, professor of psychiatry and human behavior, is currently co-leading the New England division of the U.S. POINTER study, research that aims to better represent patients impacted by Alzheimer’s (a disease that disproportionately affects older Black and Hispanic Americans). He is working with community leaders to build a trusted network to increase participation in clinical trials among people of color in Rhode Island and southern New England.
